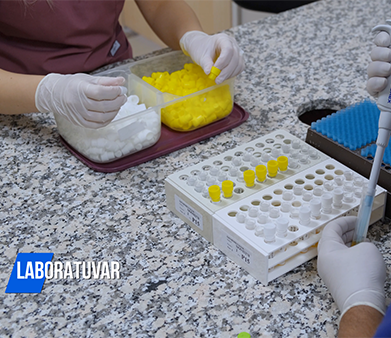
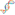What Are Clinical Trials and Why Are They Conducted?
Most clinical trials are conducted to demonstrate whether a new drug or medical device is effective and safe for use in humans.
What Are Good Clinical Practices (GCP)?
Good Clinical Practices ensure that the data obtained and reported are reliable and accurate, and that the rights, dignity, and confidentiality of research volunteers are protected in the design of clinical trials...
Why Are Clinical Trials Important?
Before new drugs and other treatments can be widely used, they must be tested to ensure they are beneficial to humans...
About Us
Farmagen Clinical
Research Center
Established in 2009 and started its services in Gaziantep University Technology Development Zone.
The Clinical Center is regularly subjected to inspections and certifications by the Ministry of Health's Pharmaceuticals and Medical Devices Agency every two years.
Bioavailability and Bioequivalence studies are conducted at the center. Over 500 projects have been completed since 2009.
Farmagen
Project Phases
With our high-quality and reliable approach, we are a preferred research center for Bioavailability/Bioequivalence studies at national and international levels.

Farmagen
Frequently Asked Questions
What do bioavailability and bioequivalence mean in clinical studies?
Bioavailability determines how effectively a drug is absorbed and enters the bloodstream. Bioequivalence measures whether different drugs deliver the same active ingredient to the body at the same rate and amount.
How is the health status of volunteers monitored in clinical studies?
The health status of volunteers is monitored using methods such as EKG, blood tests, regular check-ups, and emergency response options. All processes are overseen by a specialized team.
What data is collected during clinical studies?
Clinical studies collect pharmacokinetic (absorption, distribution, metabolism, elimination) and pharmacodynamic data to understand the effects of a drug on the body. This data provides information on the drug’s safety and efficacy.

Prof. Dr. Muradiye Nacak
Principal Investigator

Prof. Dr. Şevki Hakan Eren
Researcher

Dr. Erol Durucu
Resarcher

Dr. Taner Ezgi
Researcher

Dr. Ahmed Farah Muhumed
Researcher

Pharm. Ebru Karakuzulu
Pharmacist

Leman ERGÜL KÜLEKÇİ
Quality Assurance Manager

Mehmet Şahin
Paramedic

Ender Eşkikara
Nurse

Fuat Şahin
Paramedic

Ali Kılıç
Paramedic
Ahmet Yavuz Kaya
Nurse

Ömer Faruk Yüksek
Paramedic

Cuma Salman
Paramedic

Yakup Güçlü
Paramedic

Deniz Harman
Laboratory Technician

Abdul Halit Kılıç
Laboratory Technician

Burçin Esra Eşkikara
Laboratory Technician

Necdet Aşar
Archivist

Muhammed Eren Er
Secretary

Kemal Şahin
Auxiliary Staff

Mehmet Ali Birecik
Auxiliary Staff

Erkan Gesoğlu
Auxiliary Staff

İlyas Berktaş
Auxiliary Staff

Özlem Velieceoğlu
Auxiliary Staff
Farmagen
Our Team
Farmagen Cynic Research Center employs a Responsible Researcher, 4 Assistant Researchers, a Pharmacist, a Quality Assurance Personnel, 3 Laboratory Technicians, 6 Paramedics, 2 Nurses, 1 Archivist, 2 Secretaries and 5 auxiliary staff.
Bize Yazın